Mancozeb Reference Material
Product code: M003P250
Batch # M003B201222
Expiration date: December 22nd2023
Recommended Storage: 4°C+4°C in the dark
Weight/Vol: 250mg
Operator: GG

| Name |
Purity% |
CAS |
Raw Material |
Quantity* |
| Mancozeb |
86.0±1% |
8018-01-7 |
98431 |
253.76mg ±0.02% |
Chemistry data
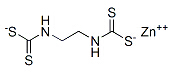
Name:Mancozeb
Formula:C4H8MnN2S4Zn
Molecular Weight: 332.71 gmol-l State: Powder Color: Grey
Melting Point:NA Boiling Point:NA Density: NA
EINECS:NA
Analysis
NMR 1H: HPLC-DAD: HPLC-FLD: GC-FID: GC-MS:
Safety Data:

Hazard statements:H317-H335-H361d-H400
Precautionary statements:P201-P273-P280-P308+P313- P333+P313-P501
This product is for routine laboratory analysis and research purposes ONLY.Not for human consumption.
GENERAL&QUALITYINFORMATION
1-General Information:
Analytical Standard Solutions-A2S is a producer of Reference Materials in neat. pure in solution and mixture forms.A2S is located in France295 Avenue de Boulac 33127 SAINTJEAN D’ILLAC.
2- Quality Standards and Documentation:
A2S is certified ISO 9001: 2015 Quality Management System-Requirements AFNOR afao G 001 Certification Number N°2012/51356.3.A2S is accredited ISO 17034 Producer of Reference Material-Reauirements COFRAC Accreditation cofrac Number N°1-6378:The scope is available on www.cofrac.fr
The certificates of analysis are designed in accordance with ISO Guide 31(Reference Materials - Contents of Certificates and labels) and ISO Guide 35(Reference Materials-General and Statistica Principal for Certification).
3-Intented Use:
The product covered by this certificate is desiened for calibration or for use in quality control rocedures for the specified chemical compound listed on the first pageThis product can be used for identification(RM)and/or quantification(CRM).This product can also be used as a reference material to validate analvtical procedures. subiect to the conditions under Section 11.For neat products quantities sold are integrate in a range between the quantity request and 5% more.If dilution is reauired use only Class A elassware and diluents compatible with all certifiedanalvtes in this preparation.Allsolutions should be thorouehly mixed prior to use.Concentration is corrected for chromatographic purity integrating,if necessary, residual water, residual solvent and residual inorganics. No adjustment is required before use.
4- Raw Materials:
Reference standards are prepared from the highest auality starting materials with defined purities. Allanalytes and solvents are obtained from pre-qualified vendors and then analyzed or evaluated prior to use.
5- Manufacturing:
All the weighing and volumetric eauipment is controlled and calibrated either by internal nrocedure on by ISO/IEC 17025 accredited calibration laboratory.
| Equipment |
Frequency |
Quality Norms |
| Weight |
Annually(ISO 17025 Lab) |
OIMLR76-1 |
| Balances |
Annually(ISO 17025 Lab)&daily (internal procedure) |
NF EN 45501 |
| Micropipettes |
Annually(ISO 17025 Lab)&monthly(internal procedure) |
NF EN ISO 8655 |
6- Homoqeneity Assessment:
Homogeneity of the finished product is assessed by analvzine sample batches or by othe method consistent with the intended use of the product and by procedures that comply with the appropriate Quality System requirementsand ISO Guide 35§7.
7- Stability Assessment:
The manufacturer guarantees the stability of this solution through the expiration date stated onthe labelwhen handled and store according to the conditions stated on the labelTo ensure a uniform solution. mix the contents of the sealed container thoroughly prior to useCare should be taken not to contaminate the contents of the original container.
8- Analytical Quality Control:
All products sold are tested by validated analvtical methods snecified in the manufacturer's quality system. Spectrums and/or chromatograms aresupplied in the way to assure the quality(identification, purity concentration)of the MR.
9- Uncertainty Statistics and Confidence Limits:
Uncertainty of the concentration is expressed as an expanded uncertaintyin accordance with ISO 17034 at the approximate 95% confidence interval usine a coverage factor of k=2 and has beer calculated by statistical analysis of our production system and incorporates uncertainty of the mass balance, purity factor,balancemicropipettes (for RM)and in addition with homogeneity and stability(for CRM).
10-Warranties:
The manufacturer warrants that its products shall conform to the description of such products as provided in its catalog or on the specific product label.This warranty is exclusive and the manufacturer makes no other warranty, express or implied,including any implied warranty of merchantability of fitness for any particular purpose.
11-Legal Notice and Limit ofLiability:
This product is for routine laboratory analysis and research purposes only. Due tothe hazardous nature, only trained personnel should handle this product.The company’s liability will be limited to the replacement of the product or refund of purchase price.Notice of claims must be made within thirty (30) davs from the date of delivery.
注:证书信息仅供参考,以产品附带证书为准。