河南普天同创计量有限公司
- 登录
-
微信公众号

- 网站地图

标准物质网
产品


- 产品
- 仪器
- 新闻
- 规程
- 帖子
- 课堂
金属混标查询
在线客服
基本信息
详细信息
相关产品
标准物质基本信息
标准物质销售提示
| 关于产品 | 所有GBW、GSB开头的标准物质,均提供“国家标准物质证书”。因产品种类繁多,普天同创官网上架的只是部分产品,详情请咨询在线客服。 |
| 关于技术 | 普天同创官网客服工作日8:30--18:00在线,欢迎随时咨询标准物质价格、标准物质证书、标准物质检测检验等相关信息。 您还可以前往普天同创官网交流区、问答区进行沟通交流。 |
| 关于发票 | 普天同创官网可自助申请开具发票,订单金额累计满5000元以上可开具增值税专用发票。 |
| 关于发货 | 普天同创拥有成熟完善的标准物质供应链,当日发货率达80%以上。 |
| 关于验收 | 买方收到产品后请立即对标准物质数量、规格型号、包装等进行标准验收。若有问题请在收到货3天内联系客服,未提出任何异议,则视为收讫。 |
相关产品
-
 冻干人血清中肌酐(中等含量)
编号:BCR-574 规格:0.9g
冻干人血清中肌酐(中等含量)
编号:BCR-574 规格:0.9g
-
 冻干人血清中肌酐(高含量)
编号:BCR-575 规格:0.09g
冻干人血清中肌酐(高含量)
编号:BCR-575 规格:0.09g
-
 肌酐冰冻人血清国家标准品
编号:360050 规格:0.5ml/支,2支/套 浓度:-70℃/避光
肌酐冰冻人血清国家标准品
编号:360050 规格:0.5ml/支,2支/套 浓度:-70℃/避光
-
 冰冻人血清尿素,肌酐标准物质
编号:GBW(E)091044 规格:1mL 浓度:详见证书,干冰运输
冰冻人血清尿素,肌酐标准物质
编号:GBW(E)091044 规格:1mL 浓度:详见证书,干冰运输
-
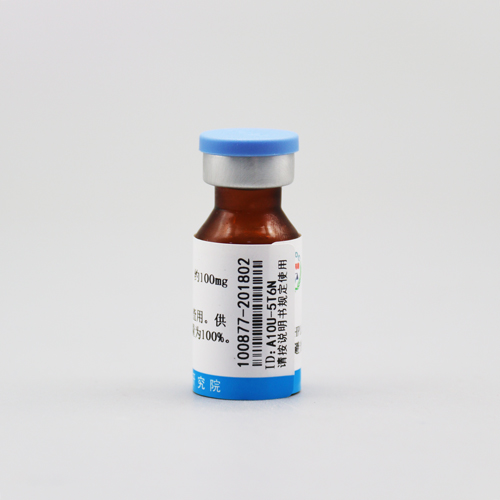 肌酐
编号:100877 CAS号:60-27-5 规格:100mg
肌酐
编号:100877 CAS号:60-27-5 规格:100mg
-
 肌酐
编号:R014148 CAS号:60-27-5 规格:25g 浓度:99%
肌酐
编号:R014148 CAS号:60-27-5 规格:25g 浓度:99%
热门产品